Previous research has looked at the use of supercapacitors as an energy storage device for portable electronics. Supercapacitors release energy in large bursts, and have incredible potential when it comes to powering our technology.
The problem is they can only be used for rapid charge/discharge cycles rather than long term energy storage.
Now a team from Drexel University has combined the properties of a supercapacitor with that of traditional batteries with large storage capacities by using a material called MXene.
"This paper refutes the widely accepted dogma that chemical charge storage, used in batteries… is always much slower than physical storage used in electrical double-layer capacitors, also known as supercapacitors," said lead researcher Yury Gogotsi from Drexel's College of Engineering.
"We demonstrate charging of thin MXene electrodes in tens of milliseconds. This is enabled by very high electronic conductivity of MXene. This paves the way to development of ultrafast energy storage devices than can be charged and discharged within seconds, but store much more energy than conventional supercapacitors."
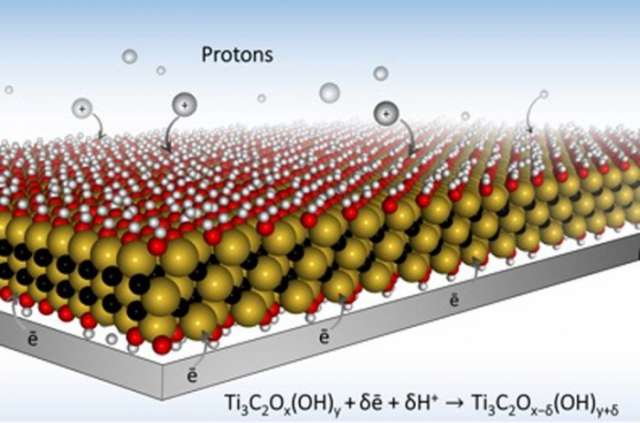
MXene is a flat nanomaterial that looks like a sandwich: consisting of oxide 'bread' with a conductive carbon and metal 'filling'. When they are made, the MXene layers stack on top of each other like Pringles.
While MXene has excellent conductivity, the Pringle structure creates a barrier that makes it tricky for ions, the chemical carriers of charge, to diffuse through the battery.
For a battery to store charge, ions are held in ports called "redox active sites". The more ports there are, the more energy the battery can hold and, importantly, the battery also has to allow the ions to move freely otherwise they can't reach the ports.
To allow the free movement of ions in MXene something had to be done about the structure.
The scientists changed the structure of the MXene by combining it with a hydrogel, turning the Pringle stack into a structure more like swiss cheese allowing the ions to flow freely.
"In traditional batteries and supercapacitors, ions have a tortuous path toward charge storage ports, which not only slows down everything, but it also creates a situation where very few ions actually reach their destination at fast charging rates," said one of the team, Maria Lukatskaya.
"The ideal electrode architecture would be something like ions moving to the ports via multi-lane, high-speed 'highways,' instead of taking single-lane roads. Our macroporous electrode design achieves this goal, which allows for rapid charging — on the order of a few seconds or less."
It's worth pointing out that while the work looks promising it's unclear exactly how the battery would be upscaled for use in a vehicle. But there's no doubt that should this research end up in cars and phones it'll completely change the way batteries are used.
"If we start using low-dimensional and electronically conducting materials as battery electrodes, we can make batteries working much, much faster than today," Gogotsi said. "Eventually, appreciation of this fact will lead us to car, laptop and cell-phone batteries capable of charging at much higher rates — seconds or minutes rather than hours."
More about: