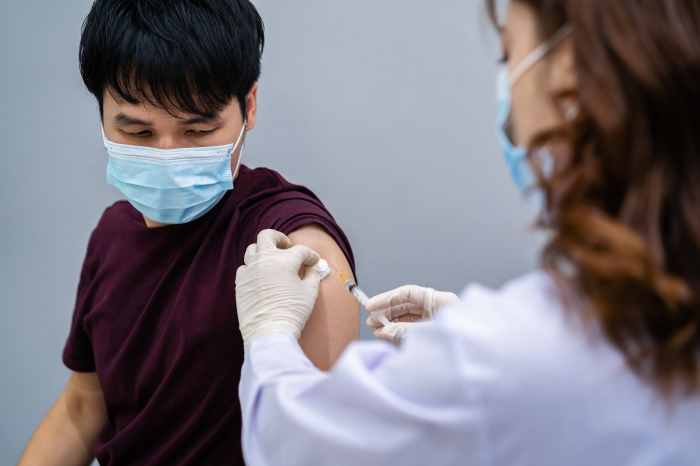
Many groups of immunocompromised people, including those with HIV, were left out of the initial clinical trials for Covid-19 vaccines.
On a hot morning in January 2022, a dozen or so women gathered in the airy, high-ceilinged reception of a health centre in Cape Town, South Africa. Seated in plastic chairs, they chatted quietly among themselves, some bouncing babies on their knees while others kept a watchful eye on toddlers playing nearby.
It was a big day for these women, who hailed from the surrounding township, Masiphumelele – they were about to enrol in a clinical trial. The intervention in question, however, wasn't entirely experimental, but one that is now a household name: Moderna's Covid-19 vaccine.
It has been more than a year since the first Covid-19 vaccines were administered and nearly 11.5 billion doses later, the available evidence suggests that they're safe and effective for most. But large populations of people were left out of the initial drug trials, excluded because they have underlying conditions such as cancer, hepatitis, kidney failure, rheumatoid arthritis, and diabetes.
Among these groups are those living with infections or illnesses that weaken their bodies' immune systems. This includes HIV, which attacks the body's immune system and can develop into the chronic, potentially fatal condition Acquired Immunodeficiency Syndrome (Aids) if untreated.
The virus affects nearly 38 million people worldwide, two-thirds of whom live in Africa. South Africa, where one in every five adults is believed to be HIV positive, is the epicentre of the continent's crisis. Townships have been particularly affected, many of which were set up under apartheid as racially segregated housing, and currently have high rates of poverty.
Scientists have suspected for some time that Covid-19 vaccines might not protect immunocompromised people as well as those with healthy immune systems. So far, studies suggest that protection rates may be slashed by as much as half for those living with HIV, but experts are now seeking a better grasp of the figures. "There is very little data about vaccine efficacy in HIV [positive] people," says Masebole Masenya, a doctor based at Masiphumelele health centre who is involved in the new trial. "We don't know how many antibodies are produced, how effective they are, or how long they last."
The urgency to find out has practical and moral drivers, in contexts where HIV is so widespread. "If you're going to roll out a Covid vaccine in Africa, it has to work in people with HIV," says physician Katherine Gill, who is in charge of the Masiphumelele test site. It's especially important to understand the vaccines' efficacy because people living with HIV are some of society's most vulnerable to Covid-19, with up to a 70% higher risk of being hospitalised from the disease than the general population.
"There's a genuine concern that there may be a reduction in vaccine effectiveness in these individuals," adds Linda-Gail Bekker, a physician-scientist and director of the Desmond Tutu HIV Centre in Cape Town. "And I would like to be able to tell them by how much. Thirty eight million people living with HIV – that's a significant group of people who deserve to know."
The new trial aims to answer these questions. How effective is the Moderna vaccine against the Omicron variant of Covid-19? Do people with HIV need more doses to be adequately protected? And how does this change if they've previously contracted the virus?
In total, the Ubuntu study, which is sponsored by the US government, will involve 14,000 people across eight African nations, 90% of whom have HIV. Enrolment began last December and preliminary results are expected by the year's end. It will compare the impact of three doses of the Moderna vaccine in people living with HIV with an identical course in those who are HIV-free. Each group contains a cohort of individuals who have previously had Covid-19 so that investigators can explore how previous infection influences immunity.
Crucially, the trial includes participants with both controlled and uncontrolled HIV, testing the vaccine's efficacy against the reality that many people may not have access to antiretroviral therapy that keeps the virus in check, or may have trouble adhering to it.
"This is probably the largest dedicated trial that is enrolling people living with HIV in the world," says Bekker. "For the first two years [of the Covid-19 pandemic], you could count on your hand how many trials there were involving HIV [positive] people."
This isn't unusual: many drug and vaccine trials exclude immunocompromised participants from initial rounds of studies for safety reasons. This is when the effects of the drug may be less well-known in humans, and the potential for harmful outcomes is higher, so giving it to people whose immune systems are already undermined could put them at greater risk. This is also partly why pregnant women and children are usually excluded from these early rounds – in addition to the potential risk of birth defects and the challenges of acquiring informed consent – though the policy has recently been questioned.
These concerns are amplified when new technologies are involved, as is the case with some Covid-19 vaccines. "There is no other licensed commercial product based on this mRNA technology. So, there is caution there," says Chris Beyrer, a professor of epidemiology at the Johns Hopkins Bloomberg School of Public Health in Maryland, who has conducted numerous studies into HIV around the world.
Pharmaceutical companies may also exclude HIV-positive participants for more strategic reasons, such as maintaining the image of a drug's efficacy. "If you [include] a lot of positive patients, and you get more breakthrough infections, that could potentially lower your vaccine efficacy…and make your vaccine less attractive," says Nigel Garrett, co-chair of the Ubuntu trial and head of HIV pathogenesis and vaccine research at the Centre for the Aids Programme of Research in South Africa (Caprisa). He speculates that the pressure to produce clean, uncomplicated results likely increased when companies like Pfizer and Moderna were in competition, vying for the high-stakes title of most effective vaccine.
Similarly, Bekker believes pharmaceutical companies may "worry what they'll do to our results, particularly where immunogenicity may be very important."
In July 2020, several HIV activist groups and research organisations in the United States protested the exclusion of HIV-positive participants from phase II and III of Moderna's vaccine trials, on the basis that when people have HIV that is well-controlled by antiretroviral therapy (ARTs) they don't qualify as "immunodeficient", because their treatment gives them comparatively normal immune function.
Additionally, the groups argued that pharmaceutical companies have an obligation to inclusively test a drug that will become a necessary, routine protection against a life-threatening disease. Moderna ultimately reversed its decision a month later and began including participants with well-controlled HIV. Soon afterwards Pfizer said it would start enrolling HIV-positive patients too.
Despite this development, Moderna's trial was still restricted to limited numbers of HIV-positive participants, all of whom had well-controlled HIV, says Garrett. The situation was similar for Johnson & Johnson's trial – HIV-positive participants numbered roughly a thousand, according to Garrett.
This was so few by large clinical trial standards that it resulted in "less than 10 endpoints, or outcomes in the study, so it was very difficult to draw any meaningful conclusions from the findings," says Garrett. "Most of the studies just didn't have people that were controlled [for HIV]...and not many took place in Sub-Saharan Africa."
This is one reason some experts believe a dedicated, large-scale clinical trial that teases out HIV's interaction with the vaccine is needed.
Beyond fairer healthcare provision for those living with HIV, part of the motivation for understanding vaccine efficacy in this group is that it could also help to prevent the emergence of new variants of Covid-19. Beyrer explains that in a healthy immune system infected with the virus, the body can clear it quite quickly. But "if your immune system isn't functioning well and you can't clear the virus, you drive it to mutate around your immune system," he says. This process of prolonged infection gives Covid a chance to try on new hats and reinvent itself, possibly transforming into a "variant of concern" – one like Delta or Omicron that is highly-infectious and virulent.
Research does show that people who are immunosuppressed – not just by HIV, but other conditions too – can accumulate multiple Covid-19 variants. In a case documented in the spring of 2020, Boston physicians discovered that a 45-year-old man living with a severe autoimmune disease had acquired more than 20 mutations over the course of five months. In another case, a 36-year-old HIV-positive woman from South Africa carried Covid for 216 days, during which the virus mutated more than 30 times.
The likelihood of mutation may also increase in HIV-positive people with low viral suppression – meaning there is more virus circulating in the body, and immunity is weakened. This describes about 30% of people living with HIV in South Africa, Garrett says. "That would be about two million people in South Africa maybe walking around with an unsuppressed viral load… that could be almost like an incubator for variants of concern."
Beyrer emphasises that there hasn't been any evidence directly linking an immunocompromised person with the emergence of a single Covid variant. And while there are millions of HIV-positive people in the world, they exist within the context of millions more – some say 2% of the world's population – who live with weakened immune systems.
In light of this, Garrett is wary of the media attention given to the idea of HIV-positive individuals as viral "reservoirs" from which Covid spreads. "We've tried to not overstate this for the last year or so, because it could result in stigma. But I think addressing this scientifically is crucial" – especially because it could help to protect this more vulnerable group.
Having these detailed clinical insights could help drive policy and allow us to employ vaccinations more effectively in service of people living with HIV.
Masenya, who was a junior doctor on the wards at the height of the Aids crisis in South Africa in the early 2000s, understands the real-world implications of poor clinical evidence. "It was terrible," he recalls. "People dying like flies because we couldn't understand HIV." This is why clinical trials are so important, he stresses, "because when people have that information, it affects policies in governments and obviously those policies affect healthcare."
One possible policy change that might follow in the wake of the Ubuntu results is the recommendation for Covid-19 boosters. South Africa currently advises that immunocompromised people receive three shots of an mRNA vaccine, or two if they've taken the Johnson & Johnson vaccine. But most Western countries, including the US and the UK, now recommend an additional dose on top of this. That's because "it really takes you up into protected levels," Beyrer says, adding that antibody levels tend to rise between 10 to 30 times with a second booster shot.
In truth, immunocompromised people might need even more than four shots, says Michelle Willicombe, a nephrologist at Imperial College London. She cites concerning data from a study conducted in France, which looked at transplant patients taking immunosuppressants to prevent their bodies from rejecting their new organs. "Just under 20% don't have any antibody responses to the fourth vaccine," she says.
To better understand the situation in the UK, where roughly half a million immunocompromised people live, Willicombe is now co-leading the Melody trial. The study began early this year and will recruit more than 35,000 patients with blood cancers and rare autoimmune diseases, or those who have received solid organ transplants. It's one of the largest ever involving this population. Researchers will study participants' antibody levels – measured by a finger prick blood test – after their third and fourth booster shots, and compare the results to hospital registries, to determine if they've had any severe Covid-19 infections or complications.
"If there is a link, it will give strength to us needing to identify those sub-populations within the immunocompromised people who don't mount an immune response, and perhaps then they require an extra level of protection," she explains.
Policymaking aside, clinical trial findings involving the immunocompromised can bring about benefits at the individual level. This is what will happen with Ubuntu, says Ivy Fikelephi Kaunda, who works with Caprisa to help recruit participants for clinical trials. Based outside the east coast city of Durban, Kaunda spends much of her time engaging on healthcare issues with residents in the surrounding townships, where over the course of the Covid-19 pandemic, she says she has encountered resistance when encouraging people to take the vaccine. "The ones who are HIV positive will turn and say 'Oh, this is not for us, because we are HIV-positive'." This fear is rooted in the fact that people living with HIV often feel like they are left out, she explains, because they are routinely told that regular treatments are not suitable for them.
But according to Kaunda, this changed with the launch of the Ubuntu trial. When, during her community rounds to recruit participants, Kaunda was able to explain that the trial was tailor-made for people living with HIV, she noticed increasing enthusiasm for the study. Now "when you go to the community people are saying 'I'm part of Ubuntu'... People understand that we also have something for them, and they'll say 'I was part of this change'."
Kaunda speculates that feeling represented by the trial might ultimately encourage more HIV-positive people to get the vaccine – which could be a positive stimulus for vaccination rates.
There's another benefit to the trial, which may play out in the longer term: knowing the precise effectiveness of a vaccine can lay a foundation for improved general healthcare, Bekker believes. "It becomes great advocacy to say to people that the vaccine works, but it's better if your immune system is fully constituted… therefore it's another reason to get tested and to take your [antiretroviral drugs]," she says. "So it's sort of a secondary public health outcome that is ideal in many ways."
Back at the health centre in Masiphumelele, the circle of women fan themselves with wads of paper – consent forms that they need to fill in before the vaccinations can begin. In the centre of the ring, a nurse explains the process, which could take most of the day and will stretch over lunch, which is cooking fragrantly in the nearby kitchen. Their willingness to devote hours of their time could perhaps be taken as an indication that, at the very least, the trial's focus on inclusion is paying off.
"In a world where we recognise that people are marginalised, stigmatised, left behind in clinical research," says Bekker, "we should do our bit to say we don't want to leave people behind."
More about: