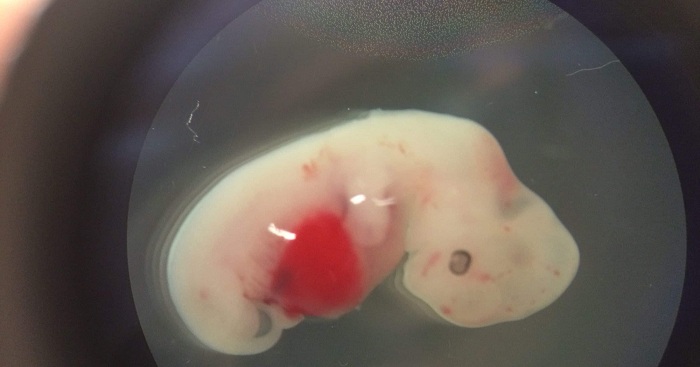
Despite this milestone, integrating cells from human and animal species is proving difficult, and developing human organs remains at a considerable distance, said Dr. Jun Wu, a staff scientist in the gene expression laboratory at the Salk Institute and first author of the research.
"Species evolve independently, and many factors dictating the developmental programs might have diverged, which makes it difficult to blend cells from one species to a developing embryo from another," Wu said. "The larger the evolutionary distance, the more difficult for them to mix."
Or as senior author Juan Carlos Izpisua Belmonte, a professor in Salk`s gene expression laboratory, sees it, "To try to imitate nature is not that easy."
Building block experiments
The project began with the research team attempting to prove that it could grow one animal`s organ cells within a different species of animal. This is known as a chimera -- specifically an interspecies chimera, an organism containing cells from two or more species.
They began with two closely related species: rats and mice.
To create a rat-mouse chimera, the scientists began by creating a mouse embryo without a pancreas.
Similar work had been done before by other scientists, notably Hiromitsu Nakauchi, now at Stanford, who bred mutant mice that lacked a pancreas and then grew a rat pancreas inside a mouse.
In the current study, Belmonte, Wu and their colleagues used gene-editing techniques known as CRISPR/Cas9 to generate mice embryos lacking a pancreas. Then they inserted rat stem cells that contained a gene for the pancreas into these mutant embryos.
Once implanted, the stem cells developed into a rat pancreas within a mouse embryo that ultimately (and importantly) grew into a healthy mouse with a normal lifespan. Taking their idea a few steps further, the researchers used the same method to develop rat eyes and rat hearts within mice embryos.
"We demonstrated the robustness of this system," Wu said. By "genetically disabling" the mouse host, they proved it was possible to generate rat organs within a host species.
Unexpectedly to the researchers, the mice delivered bonus organs after the injection of rat stem cells: gallbladders, which are present in mice but not in rats.
"This suggests that rats lack a gallbladder not because of an inherent genetic deficiency of rat cells," Wu said. Instead, "embryonic niches" may be orchestrating the tissues and organs that develop and grow within each animal species.
After the rat-mice experiments, the team turned its focus to human stem cells.
Small but significant
They began by generating different types of human induced pluripotent stem cells -- when adult cells are turned back into stem cells -- and inserting them into pig embryos. Pigs were used because both the size and the development time for their organs are more similar to our own than, say, rats.
Next, the team members implanted these embryos into sows. To test the safety and effectiveness of their work, they stopped the experiment at four weeks.
Human cells within some of the embryos had begun to specialize and turn into tissue precursors, they discovered. However, the success rate and level of human stem cell contributions in pigs was much lower than with the rat-mouse chimeras.
Wu cautioned that the research is in its very early stages, and with many challenges ahead, the development of human organs within animals may not be possible for some time.
Though the experiment with human stem cells was interrupted at 28 days, it remains the first reported case in which human stem cells have begun to grow within another species. So it is a small but significant step toward the ultimate goal of growing human organs in animals, said Insoo Hyun, an associate professor of bioethics and philosophy at Case Western Reserve University.
"They just wanted to see if the cells would survive during gestation, and they did, and they kind of migrated here and there to various sites, except the brain, which is interesting," said Hyun, who was not involved in the research.
Hyun, who worked on developing international guidelines for the International Society for Stem Cell Research, said the Salk Institute researchers appear to be staying within international ethical boundaries.
"The next step would be then to see if they can get better at localizing where the human cells develop and, ideally, getting to organ genesis," he noted.
Ethical concerns
"The interesting thing is, it was not funded by American taxpayer money," Hyun said, at least not directly. The primary funding came from private Spanish sources.
One reason for this is the US National Institutes of Health examined chimera science in a 2015 workshop and "accompanied that with a pause" on funding of a "very narrow subset of animal human chimera research," said Carrie D. Wolinetz, the institutes` associate director for science policy.
This subset consists of scientists introducing human cells into animal embryos at a very early stage.
"There has been the use of human-animal chimeras in biomedical research for a very long time. We`ve introduced human cells into animal models to create models of human diseases and used them as research tools for decades now," Wolinetz said.
"But over time, what we`ve seen -- as stem cell technology has advanced and as gene editing technology has advanced -- we`ve seen the ability to create more sophisticated animal-human chimeras at a much earlier stage of embryonic development," she said.
The reason, then, the National Institutes of Health suspended funding was over ethical concerns.
The chimeras used in biomedical research have been created, typically, by putting the human cells in at a later stage of development: at the fetal stage or even after birth. At these stages, scientists have a lot more control in that the cells stay where they are put, and they don`t "drift all over" the animal`s body, explained Wolinetz.
In these new experiments, human cells are being introduced at a much earlier stage of development, when the cells are still sorting themselves out within the embryo.
"In particular, people were concerned about human cells populating the brain of the animal or the germline of the animal," Wolinetz said. In the first case, the animal might be humanized; in the second case, the animal might pass human genes on to its offspring.
As Hyun frames it, the ethical question is this: "If you up the biological contribution of the human stem cells, are you also somehow turning them morally into a human-like thing with human rights?" He noted, though, "It`s so difficult to know how you would actually address that." It`s not measurable.
"I would argue, as long as you are avoiding the brain, or there`s not a significant change to the brain, of structure and possible functioning, then I don`t think you even need to go down that philosophical path," he said. Though as a philosopher, it might be "fun" for him, it`s not a wise or practical thing to do for policy.
Instead, Hyun said, you want to stick with measurable criteria such as seeing a drop in animal function from the beginning of an experiment to the end. "The emphasis should still remain on animal welfare," he said, as it is currently for any kind of animal research.
Meanwhile, Wolinetz said the National Institutes of Health is hoping to finalize its policy on chimera research very soon in order to get back into the business of funding appropriate projects.
"We would like to make sure exciting research can go forward," she said, "but also make sure that we`ve got an appropriate oversight system so we can make sure it`s proceeding responsibly."
More about: