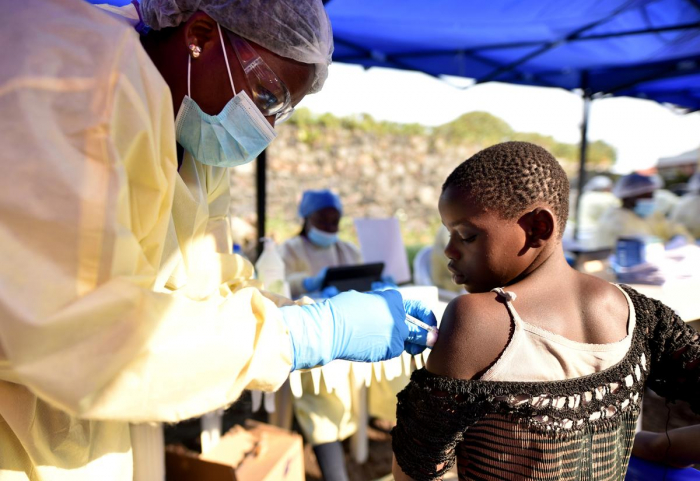
Oly Ilunga, who opposed using the vaccine developed by U.S. pharmaceutical giant Johnson & Johnson, resigned as minister on Monday after being bumped off the Ebola response team.
The World Health Organization recommended the two-dose shot to complement a vaccine by U.S. drugmaker Merck, which has proved highly protective but is in relatively short supply.
Proponents, including medical charity Medecins Sans Frontieres (Doctors Without Borders) and the Wellcome Trust, said the new vaccine could be deployed to areas not yet affected by Ebola to create a firewall against the virus, which the WHO declared an international health emergency last week.
But Ilunga said the J&J vaccine had not been proven effective and could confuse people in eastern Democratic Republic of Congo, where wild rumours are hampering the response.
“Congolese have the right to have the gold standard, the best vaccine,” he told Reuters on Thursday, in his first public comments since resigning. “They don’t need to be the subject of experimentation.”
“You can’t have a group of promoters, producers of the vaccine (and) university researchers wanting to introduce the vaccine without contacting the health authorities,” he said, without elaborating further.
Paul Stoffels, J&J’s chief scientific officer, denied there were any efforts to secretly introduce the vaccine and said the company had been in full communication with Congolese authorities.
But scepticism about new medicines can resonate strongly on a continent where some pharmaceutical trials have faced accusations in the past of failing to obtain informed consent and providing subpar care to participants.
For example, some U.S. government-funded trials of HIV drugs in the 1990s were accused of double standards for giving placebos to women in Africa when effective therapies existed, a practice that is not generally allowed in the United States and other Western nations on ethical grounds. Researchers defended the use of placebos as scientifically necessary.
Jean-Jacques Muyembe, an epidemiologist and Ebola expert named to lead Congo’s response team, dismissed Ilunga’s concerns and said authorities would revisit whether to deploy a second vaccine. However, he downplayed the importance of the decision.
“I don’t think that a vaccine is what’s holding back the response,” he told Reuters, noting that previous Ebola outbreaks had been contained quickly without a vaccine.
“We could use or not use. It won’t change the evolution of the epidemic,” he said.