By Tom Garmeson
Research suggests some parts of the body age faster than others to the point that they even outlive their owners. Could understanding this better help us to live longer?
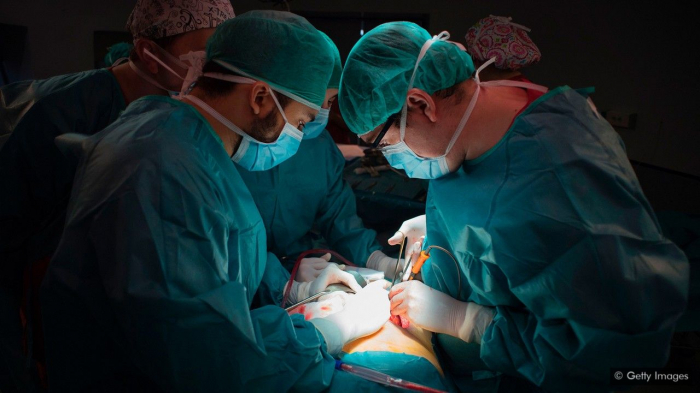
It was a desperate situation. A 19-year-old Turkish woman with liver disease was in urgent need of a transplant. While on the waiting list, she developed hepatic encephalopathy, a condition where toxins building up in her bloodstream due to her failing organ began to take their toll on her brain.
Before long, her liver started to shut down altogether and doctors rushed to save her life.
With time ticking away, their only option was a liver that had already been turned down by other hospitals. It was considered to be in bad shape – not only did it contain a cyst caused by a parasitic infection, but its previous owner was a recently deceased 93-year-old woman. The organ was old by transplant standards, particularly for a recipient so much younger.
But with no other organs available and little other choice, the doctors went ahead with the transplant. Remarkably, the operation, which took place in 2008 at the Liver Transplantation Institute at Inonu University in Malatya, Turkey, was a success – the young recipient survived, and six years later gave birth to a healthy baby girl. On her daughter’s first birthday, the woman had turned 26 and had just celebrated her liver’s one-hundredth birthday.
Few of us will ever know what it’s like to have a liver as old as our great-grandparents. But remarkably some of our organs have the capacity to outlive us, while others age far more quickly. The way your organs and tissues age could tell us far more about how old our bodies really are than counting birthdays ever will.
One of the curiosities of longevity research is that your actual age appears to be less important than you might expect. In fact, researchers tend to be more interested in the discrepancy between your chronological age – how many years since you were born – and your biological age, a concept used to describe how our bodies are actually faring as we get older.
These two numbers may be linked, but it makes intuitive sense that they don’t always match up. We are all well aware that a lifetime of unhealthy eating and lack of sleep will age us prematurely.
Ageing is often thought of as a gradual process that happens to the body as a whole, albeit at different speeds for different people. But while it can be helpful to examine our decline at a holistic level, doing so may not provide us with the whole picture. Research shows that the complex mix of genetic, lifestyle and environmental factors that determine how quickly our bodies age does not affect all our organs in equal measure.
So, while we may have the youthful appearance of a 38-year-old, our kidneys might have the shrivelled appearance of one from a 61-year-old, as one study in adults found. Equally we could have all the wrinkles and hair loss of an 80-year-old, but still have the beating heart of a 40-year-old.
Stanford University geneticist Michael Snyder likens it to a car. “Over time, the whole functioning of a car declines, but some parts wear out faster than others,” he says. “If your engine is starting to go you can fix that – if later the body wears out you can fix that, and so on.”
So, while knowing our aggregate biological age is useful, if we want to live longer, healthier lives, we should also pay attention to the fact that not all of our body parts age alike.
But accurately estimating the biological age of any organ is not a simple task. While many websites offer “calculators” to help estimate the age of different organs like the heart or lungs, it really requires detailed examination of the organ’s function, tissue structure, cellular make up and genetic health to assess it accurately.
Transplant data provides some intriguing clues as to which organs might fare better with age. Researchers comparing the age of donors with factors such as how long the recipient lived post-surgery found that across the board transplants tended to be less successful with older organs. There were, however, significant differences depending on the organ, suggesting some body parts hold up better with age.
Whereas the success rate with hearts and pancreases worsened after the age of 40, the researchers couldn’t spot any age-related differences with transplanted lungs until the donors were over 65. Corneas were the most resistant organ of all, with donor age apparently having little effect.
The researchers, based at the University of Liverpool, UK, suggest that the relative complexity of the organs, along with their reliance on blood vessels to function, were probably key factors in how they coped with age. “It is logical to think that age-related changes in vasculature and microvasculature of the different organs must be a significant factor contributing to their age-related dysfunction,” they wrote.
The transplant data also raises questions about whether there is an upper limit on the lifespan of certain organs. The liver, for example, is well known for its regenerative capabilities, and patients who have had up to two thirds of their liver surgically removed can find the organ almost completely regrows to its previous size within a year. Some researchers have suggested that nonagenarians are a largely untapped pool of potential liver donors, citing several successful transplants in recent years. Others are monitoring a select club of transplant patients whose livers have turned one hundred several decades before they will.
Certain organs may be more sensitive to some aspects of our lifestyle than others. “A very good example is the lungs and pollution,” says Richard Siow, director of ageing research at King’s College London. “Lungs are more aged in the city, or in high pollution environments.”
According to Siow, any number of lifestyle factors can influence our complex patterns of ageing. “What we eat and how we eat it, how we sleep and when we sleep – all these things can impact our organs in varying ways that we don’t fully understand,” he says.
At the microscopic level, the concept of an organ’s age becomes fuzzier still. The individual cells making up most of our organs wear out and require replacement on a fairly regular basis, meaning many tissues completely regenerate over time, but the rate at which they do so varies hugely. A red blood cell circulates in your veins and arteries for an average of four months, while those cells braving the rough and tumble of your gut need to be replaced after just a few days. At the other extreme, most brain cells, or neurons, are not replaced as we age, meaning they are generally as old as the body itself.
But in 2019, a team led by Martin Hetzer at the Salk Institute of Biological Studies were surprised to discover that neurons were far from the only cells in mammals that boasted a long lifespan. They found cells in the liver and pancreas of mice that were as old as the animals themselves and were coexisting with much younger cells – an arrangement known as “age mosaicism”. Since long-lived cells are more vulnerable to age-related wear and tear than those that only last a few days, the fact they are present outside the brain could give researchers clues as to the ageing mechanisms of these other organs.
Personalised ageing
Regardless of how resilient our organs are in the face of ageing, all of them will gradually slow down over time, but new research suggests we may be able to predict which ones will be the first to go.
In 2020, Snyder, Wenyu Zhou, Sara Ahadi and colleagues at Stanford University identified at least 87 molecules and microbes present within the body that could be used as “biomarkers” of ageing. By examining how these markers shifted in a group of volunteers over several quarterly check-ups for two years, the team found that people appeared to be ageing via different biological mechanisms. What’s more, they found they could categorise individuals into different “ageotypes”, by grouping the biomarkers based on which organ or system they were most closely associated with.
The team found evidence for four different types of ager, based on their dominant ageing pathway – kidney, liver, metabolic and immunity – but believe that others, such as cardio agers, exist too. Notably, they were able to identify people’s ageotype, which Snyder says is likely down to a combination of genetic and environmental factors, long before they reached old age. If the Stanford team are right, young people might one day be told which aspect of their health they need to look out for as they get older.
“If you are a cardioager, make sure you watch your bad cholesterol, get your heart checked, exercise,” says Snyder. “For a metabolic ager, watch diet; liver agers, drink less alcohol, etc.”
Critics point out that we don’t yet know whether the ageotypes identified by the Stanford team in their relatively short study will actually result in physiological changes that negatively impact health in the longer term. But Snyder is certain we are entering an age of more personalised approaches to anti-ageing interventions. “One-size-fits-all doesn’t cut it,” he says. “Exercise and good diet can help overall, but if your heart or kidney is wearing down you may need to do more directed strategies.”
Winding back the clock
Recent advances in machine learning are allowing scientists to gain more precise estimates of biological ageing too. One of the approaches involves studying semi-permanent changes to our DNA known as methylation. Various genes are switched on or off by this process, which involves a methyl chemical group attaching to parts of our DNA. It is thought that this process is one of a number of “epigenetic” changes to our DNA that determine how our genes are influenced by our lifestyle and the environment we live in. The amount of DNA methylation varies as we get older and our epigenetic patterns change. This has allowed biologists to develop “epigenetic clocks”, which they believe are powerful predictors of biological age.
The clocks also allow researchers to compare the biological ages of different tissues. There is some evidence that female breast tissue, for example, ages faster than the rest of the body, raising questions about whether epigenetic clocks could be used as a predictor of breast cancer.
Even if they prove to be accurate, some scientists note that we don’t yet know whether treatments that slow these clocks down will also slow the ageing process.
Whichever way we look at ageing, the ultimate goal for many a longevity researcher is not just to slow down the clock, but actually reverse it. At the cellular level, this already seems to be possible. In March 2020 researchers from the Stanford University School of Medicine said they had managed to rejuvenate cells taken from elderly people by making them produce Yamanaka factors, proteins which have previously been shown to wind cells back to an embryonic state. After a few days of this process, the cells appeared years younger.
Doing something similar to a whole organ may be somewhat more challenging, but the research could be the first step towards new treatments that could turn back the biological clock of cells and tissues without removing them from the body.
For now, however, many scientists are focussed on extending the healthy lifespan – healthspan – of elderly people. A recent review paper by Linda Partridge and colleagues at University College London highlighted drugs such as rapamycin, metformin, and lithium as potentially promising treatments to delay the onset of diseases and problems that accompany old age, but noted that none of these interventions came close to reversing all of ageing’s numerous symptoms. Others agree that anti-ageing treatments will probably have “tissue-specific” effects, highlighting the need for scientists to understand how the ageing process affects different organs in different ways.
However many differences there may be in the way each organ ages, it makes sense to look after them all. Richard Siow stresses that they are still interconnected systems and that the ageing of one will inevitably affect the others.
“If you have inflammation in your joints, that inflammation is going to impact on your brain and your heart as well,” he says. “Every different organ has a different ageing trajectory, but it’s all interrelated”.
BBC
More about: #Organs