Back in the 1990s, the hole in the planet's ozone layer was a pressing global crisis – if we had ignored it, today there would be several.
In the late 1970s, Jonathan Shanklin, a meteorologist with the British Antarctic Survey, spent much of his time tucked away in an office in Cambridge working through a backlog of data from the southernmost continent on our planet.
Shanklin was responsible for supervising the digitisation of paper records and computing values from Dobson spectrophotometers – ground-based instruments that measure changes in atmospheric ozone.
As the years passed, Shanklin started to see that something was going on – after nearly two decades of fairly constant measurements, he noticed that ozone levels began dropping in the late 1970s. Initially, Shanklin’s bosses weren't as certain as he was that something was happening, which frustrated him.
By 1984, the ozone layer over Antarctica's Halley Bay research station had lost one-third of its thickness compared to previous decades. Shanklin and colleagues Joe Farman and Brian Gardiner published their findings the following year, suggesting a link to a human-made compound called chlorofluorocarbons (CFCs), used in aerosols and cooling devices. Their discovery, the thinning of the ozone layer over Antarctica, came to be known as the ozone hole.
As news of the discovery spread, alarm rippled around the world. Projections that the destruction of the ozone layer would adversely impact the health of humans and ecosystems sparked public fear, mobilised scientific investigation and galvanised the world’s governments to collaborate in an unprecedented way.
Since its heyday, the story of one of the gravest environmental problems that humanity has faced has largely fallen from the radar.
More than 30 years on from its discovery, what ever happened to the hole in the ozone layer?
A vital phenomenon
Ozone is mostly found in the stratosphere, a layer of the atmosphere between six and 30 miles (10-50 km) above the Earth's surface. This ozone layer forms an invisible protective shield over the planet, absorbing damaging UV radiation from the sun. Without it, life on Earth would not be possible.
The British Antarctic Survey first began measuring ozone concentrations above Antarctica in the 1950s. But several decades passed before it became clear there was a problem.
In 1974, scientists Mario Molina and F. Sherry Rowland published a paper theorising that CFCs could destroy ozone in Earth's stratosphere. Until then CFCs were thought to be harmless, but Molina and Rowland suggested that assumption was wrong. Their findings were attacked by industry, who insisted their products were safe. Among scientists, their research was contested. Projections indicated that ozone depletion would be minor – between 2-4% – and many thought it would happen on a timescale of centuries.
The use of CFCs continued unabated and by the 1970s they were ubiquitous worldwide, used as coolants in refrigerators and air conditioners, in aerosol spray cans and as industrial cleaning agents.
A mere decade later, in 1985, the British Antarctic Survey confirmed a hole in the ozone layer and suggested a link to CFCs – vindicating the work of Molina and Rowland, who were eventually awarded the 1995 Noble Prize in chemistry. Even worse, the depletion was happening much quicker than had been anticipated. "It was really quite shocking," says Shanklin, now an emeritus fellow at the British Antarctic Survey.
From then on, scientists raced to figure out how and why this was happening.
n 1986, as the Antarctic winter neared its end, Susan Solomon, a researcher with the US government National Oceanic and Atmospheric Administration, led a team of scientists to McMurdo Base in search of answers. At the time, scientists were debating three possible theories, one of which Solomon had proposed: that the answer might lie in surface chemistry involving chlorine on polar stratospheric clouds, which occur at high latitudes and only form during very low temperatures in polar winter.
"It was a great mystery," says Solomon, now professor of atmospheric chemistry and climate science at MIT. Her research explained how and why the ozone hole occurs in Antarctica. "All the data pointed towards the combination of the increase of chlorine from the human use of CFCs and the presence of polar stratospheric clouds as being the trigger for what happened."
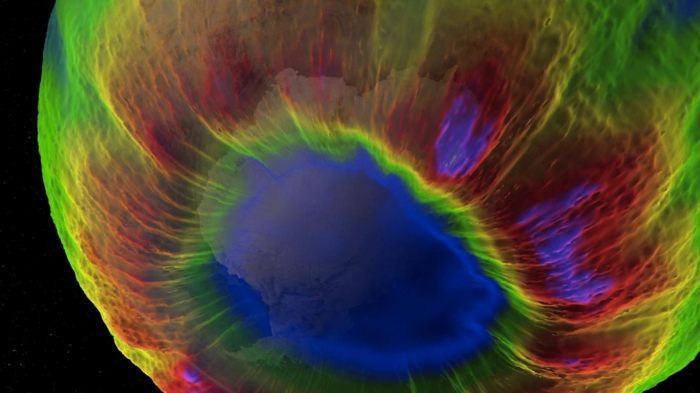
Satellite monitoring confirmed ozone depletion extended over a vast region – 7.7 million square miles (20 million sq km).
The serious threat posed by ozone depletion – rises in skin cancer and cataracts in humans, harm to plant growth, agricultural crops and animals and reproductive problems in fish, crabs, frogs and phytoplankton, the basis of the marine food chain – spurred international action and collaboration.
But considering how grave a threat the ozone hole was deemed to be, why do we not often hear about it anymore?
"It's not the same cause for alarm that it once was," says Laura Revell, associate professor of environmental physics at the University of Canterbury, New Zealand. This is largely due to the unprecedented international steps that governments took to tackle the problem.
Thinking ozone depletion would be small and far into the future, international policymakers initially took a cautious approach to ozone protection. In 1977, a global action plan was adopted, calling for monitoring of ozone and solar radiation, research on ozone depletion’s effect on human health, ecosystems and the climate and a cost-benefit assessment of control measures. A few months before the discovery of the ozone hole by the British scientists, this led to the 1985 Vienna Convention, calling for further research. But it didn't include legally binding controls for CFC reduction, disappointing many.
After the ozone hole discovery, heavy investment in scientific research, marshalling of economic resources and coordinated international political action helped to turn things around.
In 1987, the Montreal Protocol was adopted to protect the ozone layer by phasing out the chemicals which deplete it. To support compliance, the treaty recognised "common but differentiated responsibilities", staggering phase-out schedules for developed and developing countries and establishing a multilateral fund to provide financial and technical assistance to help developing countries meet their obligations.
During the 1990s and early-2000s, the production and consumption of CFCs was brought to a halt. By 2009, 98% of the chemicals agreed to in the treaty had been phased out. Six amendments — which the treaty allows when scientific evidence shows further action is needed — have led to ever-tightening restrictions on substances introduced to replace CFCs, such as hydrochlorofluorocarbons (HCFCs) and hydrofluorocarbons (HFCs). While good for the ozone layer, these replacements turned out to be bad for the climate. The global warming potential of the most commonly used HCFC, for example, is almost 2,000 times stronger than carbon dioxide.
The treaty's climate benefits have been a positive side effect. In 2010, emissions reductions due to the Montreal Protocol were between 9.7 to 12.5 gigatons of CO2 equivalent, approximately five to six times more than the target of the Kyoto Protocol, an international treaty adopted in 1997 that aimed to reduce greenhouse gas emissions. The 2016 adoption of the Kigali Amendment, which will limit the use of HFCs, will help avoid up to 0.5 C of global warming by 2100.
"You could argue [the Montreal Protocol] is a much more successful bit of climate protection legislation than any of the other [climate] agreements we've had to date," says Revell.
Since its adoption, the Montreal Protocol has been signed by every country on Earth – to date the only treaty to be universally ratified. It's widely considered a triumph of international environmental cooperation. According to some models, the Montreal Protocol and its amendments have helped prevent up to two million cases of skin cancer yearly and avoided millions of cataract cases worldwide.
Had the world not banned CFCs, we would now find ourselves nearing massive ozone depletion. "By 2050, it's pretty well-established we would have had ozone hole-like conditions over the whole planet, and the planet would have become uninhabitable," says Solomon.
Solomon credits three factors for the swift action on the problem: the clear and present danger the ozone hole posed to human health made it personal to people, vivid satellite imagery made it perceptible and there were practical solutions to it – ozone-depleting substances could be replaced fairly quickly and easily.
A long recovery
Today, the ozone hole still exists, forming every year over Antarctica in the spring. It closes up again over the summer as stratospheric air from lower latitudes is mixed in, patching it up until the following spring when the cycle begins again. But there’s evidence it’s starting to disappear – and recover more or less as expected, says Solomon. Based on scientific assessments, the ozone layer is expected to return to pre-1980 levels around the middle of the century. Healing is slow because of the long lifespan of ozone-depleting molecules. Some persist in the atmosphere for 50 to 150 years before decaying.
Despite the Montreal Protocol's overall success, there have been setbacks. In 2018, for example, the concentration of CFC-11, banned since 2010, was found to not be coming down as quickly as was expected, suggesting undeclared emissions were coming from somewhere. The Environmental Investigation Agency traced the emissions to factories in China, which were manufacturing it for use in insulation foam. Once made public, the Chinese government quickly clamped down and scientists say we are now back on track.
For Shanklin, this underlines the vital importance of long-term monitoring of environmental variables, whether CFCs, temperature or biodiversity indicators. "If we're not monitoring them then we don't know whether we're in trouble or not, and if you don't know you're in trouble, you can't take preventative action and I think that's a vital part of this story."
And the future is not without risks. Major volcanic eruptions typically result in short-term ozone losses, while nitrous oxide, a powerful greenhouse gas emitted from fertiliser applications in agriculture, is also a potent ozone-depleting substance. However, it's not controlled by the Montreal Protocol, explains Revell – and emissions are growing.
There are also activities whose impact we don't yet fully understand but might pose risks, like rocket launches and sulfate geo-engineering – the idea we can stave off the worse effects of global warming by pumping aerosol into the stratosphere to cool the climate, by causing sunlight to be reflected off those aerosol particles.
"It's really important we do keep in mind the lessons learned from the ozone hole story and make sure we're constantly aware of what's going on in the stratosphere," says Revell. "The risk is we cause some unforeseen damage to the ozone layer if such assessments are not carried out in advance."
There’s a tendency to compare the ozone hole to climate change, yet while the Montreal Protocol does demonstrate we can tackle large environmental problems the comparison only goes so far. CFCs were a replaceable component of a few products. The scope of climate change makes it considerably more difficult to address; fossil fuels are pervasive throughout our lifestyle, they cannot be replaced nearly as easily and most governments and industry have, thus far, resisted reducing fossil fuel emissions.
For Shanklin, it's sad to have wound up where we are, stalled on climate action, still talking about what we might do, when there's such a clear example to learn from.
"The creation of the ozone hole showed how rapidly we can change our planetary environment for the worse and that lesson is not really being taken seriously enough by the politicians," says Shanklin. "Climate change is a bigger problem, to be fair. But that doesn't absolve the politicians of responsibility for making the necessary decisions."
BBC
More about: