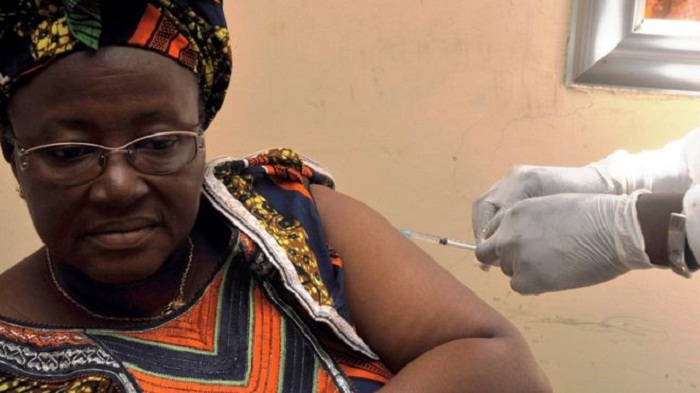
The vaccine is now being fast-tracked for regulatory approval.
Manufacturer Merck has made 300,000 doses of the rVSV-ZEBOV vaccine available for use should Ebola strike.
GAVI, the global vaccine alliance, provided $5m for the stockpile.
Results, published in The Lancet medical journal, show of nearly 6,000 people receiving the vaccine, all were free of the virus 10 days later.
In a group of the same size not vaccinated, 23 later developed Ebola.
Only one person who was vaccinated had a serious side effect that the researchers think was caused by the jab. This was a very high temperature and the patient recovered fully.
It is not known how well the vaccine might work in children since this was not tested in the trial.
More about: