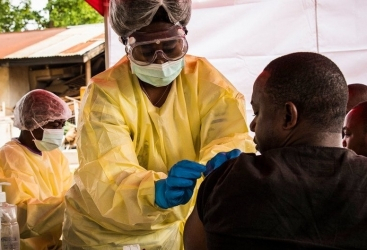
The recommendation follows review and analysis of clinical trials for the monoclonal antibodies mAb114 (known as Ansuvimab or Ebanga) and REGN-EB3 (Inmazeb), which have demonstrated clear benefits for people who have tested positive for Ebola, which is often fatal.
This includes older persons, pregnant and breastfeeding women, children, and newborns whose mothers were confirmed to have Ebola within the first seven days after birth.
The clinical trials were conducted during Ebola outbreaks. WHO said the largest trial was carried out in the Democratic Republic of the Congo, demonstrating that the highest level of scientific rigour can be applied even during Ebola outbreaks in difficult contexts.
The UN agency also provided recommendations regarding therapeutics that should not be used as treatments, which include ZMapp and remdesivir.
The new guidance, published simultaneously in English and French, will support healthcare providers caring for Ebola patients as well as policymakers involved in outbreak preparedness and response.
It complements clinical care guidance that outlines the optimized supportive care that Ebola patients should receive – from the relevant tests to administer, to managing pain, nutrition and co-infections, and other approaches that put patients on the best path to recovery.